Periodic Spiral Table : Elements
Periodic Spiral Table
The Elements of the periodic table is the basis fotr all chemistry, electricity and material that we encounter on a familiar and daily basis. However the nature of these matters are surely stimulating the soul on the deepest level. An example of extraordinary beauty and complicated designis to see for instance a Bismuth Crystal, look it up and be thrilled !
The idea on this spiral design came from the investigation wether soft Iron that is more mallable and workable, and also have increased Magnetic respons. Thus being favoured in electrical generator coils. Wanting to buy soft Iron is a challenge. the industry wants Hard Iron that can take alot of wheight, not Iron. Hard Iron was in the old days wrought in a furnace and in that process of being heated adding coal paricles. Becoming Steel. steel is Hard Iron. Becoming Hard Iron the Fe26 becomes "contaminated and less Magnetically permable as it is called.
I asked myself if Stainless Steel that is another version of Iron Fe26 and C6 Carbon had such an amount of Carbone that the Oxygene O8 in the water and atmosphere did not React with the Iron, becoming Rust or Oxidized ? It was not so the stainless Steel is an alloy that has more atomic elements than just Carbon. It consist of Chromium 24, Manganese 25, Silicon 14 and Nickel 14. these metals form a "seal" over the Iron atoms not allowing the Oxygene that is a strong Acid atom to react and Oxidize forming Rust on the Iron atom. Oxygene is a greek word meaning OXY (sour) and Genes gender . Oxygene is very electron rich in its placement in the octet of the periodic table.
The Octet Rule.
The Electrons are in their nature Negative, and the Nucleus UUD is in their nature of opposite polarity +. Making the atom a magentic Dipole. dipole means 2 - poles, North and South considered a "magnet pair". when the electrons are rubbed away from the Atom, it becomes Charged, that is known in Triboelectric Charge when for instance a PVC pipe is charged (+) poisitve by rubbing a cloth onto it. the electrons are removed, leaving the Atoms in need of a need for balance.
The Atom Octet has an "invisible desire" that in the desires a 2, 8, 18, or 32 electrons in the outer shells. when the innermost electron shells are stabile. The different octet domains are 2 electrons in the outer shell, or 8 in the next 18, then 32 electrons in the outermost shell. Lithium for example has 3 protons and 3 neutrons but still desires 8 Electrons in its outer shell. the Atoms can (seemingly) interact in reactions where all the 8 electrons are utilized in a combination with other atoms, forming a molecule. This is called the Covalent Bond theory and well established in the chemical community.
I am still investigating Molecules and how the charges of electrons apply to the Protons. It will take some time. so before all that is in place I just print the Spiral table to get som comments on it.
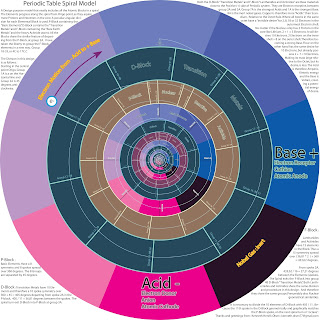
I was very proud of it at once, then soon realizing it is made a spiral Periodic system back in the days by Ingo Waldemar Dagobert Hackh 1914. so this is nothing new. However I have seen so many "periodic systems so this is but a new interpreteion. I will keep more coming when I find something interesting to write. The basis of this investigation is to find out what way the Electrons move in a DC battery. Do they go from + to -. Or from negative - to positive + ? This particular model is more concerned with the Atoms and the electrons. It is more complex when it comes to molecules. and it will take more time to investigate.
The Spiral Periodic System.
It seemed reasonable to figure a way to make the Atoms progress along a spiral as the proton mass increased. the Groups are arranged in spokes from the center.
Bigger image : http://imageshack.us/photo/my-images/13/4aox.jpg/
The Rendering is in Low resolution. But the idea is visible.
This is a early version. Write an Email and I will be happy to send a full resolution bitmap file.
The idea presented itself to me when I wanted to draw a spiral version
of the Period System. I drew the basic elemets on paper, then I saw
the limitations of a 8 symmetry. when Iron shurely did not go into group 8 along with the inert gases. The 8 symmetry had to depart at Calcium and enter a new "domain" Scandium. I expanded the design in a special way and suddenly I saw a large system unfold. Including the Actanides and the Lanthanides in a spiral system that did not have the loops or additional rows of elements in the classical designs. This design seems to function in a good way, it looks very neat and hopefully of inspiration. Here is what I have.
Kenneth Henrik Olsen.
Oslo 10.7.2013
---
Periodic Table Spiral Model.
A Design purpose model that neatly includes all the blocks
in a spiral expanding from a origo point.
In a peculiar angular division for each block secion.
All the Blocks depart from the P-Block at group 2A.
The division is as follows :
In this design Group 1A is a on the Horizontal line
and Group 2A is 45 degrees anticlockwise.
P-Block : Basic Elements. Have a 8 symmetry and 8 spokes on 360 degrees. Groups are separated by 45 degrees.
D-Block : Transistion metals have 10 symmetry on 360 + 45 = 405 degrees
from spoke 2A. 405 / 11 = 36,81 degrees between the spokes.
and spiral turn exit D block into P-block group 3A.
F-Block : Lanthanides and actinides have a 15 elements
thus a 15 symmetry spread over (36,81*2 ) + 360 = 433,62 degrees
From spoke 2A. 433,62 / 16 = 27,21 degrees between the elements (spokes).
The Spiral exits the F-Block into group 4B transition metals.
Both Lanthernides and Actinides share the same division and proceedure in this design. That is they share the same groups. A misunderstanding led me
to write Landanoids instead of Lanthanoids. I thought the name related to "Land" since it
is Rare Earth Metals. Now knowing that Lanthnoids
are greek from "hidden elements".
It is neccesary to divide the 10 elemetns of Fblock with
405 / 11, because the 11th spoke in the D-Block geometrically and graphically matches the p-block spoke, on the next spiral turn or "octave".
the same with the F-blocks that are 15 elements
they are divided by number 16. 433.62 / 16. the 16th is the "0th" spoke
on the next block, or d-block
---
The elements progress along the spiral as they aquire more
Protons and Neutrons in the core. The smalles atomic size
is therefore near the origo or central point of the spiral.
Ideally the Hydrogene and Helium should have their own 2 fold symmetry.
In this design the H and He share the 8 spoke symmetry of the P-block.
I Have taken the liberty to change the Group names for the
Heavier elements d-Block. 4C - 17C or group 19 - 33.
There is also a description on the Bases and Acids.
And a attempt to clearify the misunderstanding
that the Ampere moves from the + terminal of the battery toward the -.
Load up the drawing and start the viewing from the central Origo.
The spiral will take you through the complete Periodic System !
Have a good one
Here is the work file where the numbers are arranged
Proton : Electrons New CAS IUPAC Group
H 1 : 1 Group 1 Group 1A 1
He 2 : 2 Group 2 Group 2A 2
Li 3 : 2 - 1 Group 1 Group 1A 1
Be 4 : 2 - 2 Group 2 Group 2A 2
B 5 : 2 - 3 Group 3 Group 3A 13
C 6 : 2 - 4 Group 4 Group 4A 14
N 7 : 2 - 5 Group 5 Group 5A 15
O 8 : 2 - 6 Group 6 Group 6A 16
F 9 : 2 - 7 Group 7 Group 7A 17
Ne 10 : 2 - 8 Group 8 Group 8A 18
Na 11 : 2 - 8 - 1 Group 1 Group 1A 1
Mg 12 : 2 - 8 - 2 Group 2 Group 2A 2
Al 13 : 2 - 8 - 3 Group 3 Group 3A 13
Si 14 : 2 - 8 - 4 Group 4 Group 4A 14
P 15 : 2 - 8 - 5 Group 5 Group 5A 15
S 16 : 2 - 8 - 6 Group 6 Group 6A 16
Cl 17 : 2 - 8 - 7 Group 7 Group 7A 17
Ar 18 : 2 - 8 - 8 Group 8 Group 8A 18
K 19 : 2 - 8 - 8 - 1 Group 1 Group 1A 1
Ca 20 : 2 - 8 - 8 - 2 Group 2 Group 2A 2
Transition metals : d-block
Sc 21 : 2 - 8 - 9 - 2 Group 9 Group 3B 3
Ti 22 : 2 - 8 - 10 - 2 Group 10 Group 4B 4
V 23 : 2 - 8 - 11 - 2 Group 11 Group 5B 5
Cr 24 : 2 - 8 - 13 - 1 Group 12 Group 6B 6
Mn 25 : 2 - 8 - 13 - 2 Group 13 Group 7B 7
Fe 26 : 2 - 8 - 14 - 2 Group 14 Group 8B (a) 8
Co 27 : 2 - 8 - 15 - 2 Group 15 Group 8B (b) 9
Ni 28 : 2 - 8 - 16 - 2 Group 16 Group 8B (c) version 1 10
Ni 28 : 2 - 8 - 17 - 1 Group 16 Group 8B (c) version 2 10
Cu 29 : 2 - 8 - 18 - 1 Group 17 Group 1B 11
Zn 30 : 2 - 8 - 18 - 2 Group 18 Group 2B 12
Basic elements
Ga 31 : 2 - 8 - 18 - 3 Group 3 Group 3A 13
Ge 32 : 2 - 8 - 18 - 4 Group 4 Group 4A 14
As 33 : 2 - 8 - 18 - 5 Group 5 Group 5A 15
Se 34 : 2 - 8 - 18 - 6 Group 6 Group 6A 16
Br 35 : 2 - 8 - 18 - 7 Group 7 Group 7A 17
Kr 36 : 2 - 8 - 18 - 8 Group 8 Group 8A 18
Rb 37 : 2 - 8 - 18 - 8 - 1 Group 1 Group 1A 1
Sr 38 : 2 - 8 - 18 - 8 - 2 Group 2 Group 2A 2
Transition Metals : d-block
(Next to outer electron shell is increasing, outer is stabile at 1 electron)
Y 39 : 2 - 8 - 18 - 9 - 2 Group 9 Group 3B 3
Zr 40 : 2 - 8 - 18 - 10- 2 Group 10 Group 4B 4
Nb 41 : 2 - 8 - 18 - 12- 1 Group 11 Group 5B 5
Mo 42 : 2 - 8 - 18 - 13 -1 Group 12 Group 6B 6
Tc 43 : 2 - 8 - 18 - 13 -2 Group 13 Group 7B 7
Ru 44 : 2 - 8 - 18 - 15 -1 Group 14 Group 8B (a) 8
Rh 45 : 2 - 8 - 18 - 16 -1 Group 15 Group 8B (b) 9
Pd 46 : 2 - 8 - 18 - 18 Group 16 Group 8B (c) 10
Ag 47 : 2 - 8 - 18 - 18 - 1 Group 17 Group 1B 11
Cd 48 : 2 - 8 - 18 - 18 - 2 Group 18 Group 2B 12
Basic elements : P-block
( outer electron shell is increasing)
In 49 : 2 - 8 - 18 - 18 - 3 Group 3 Group 3A 13
Sn 50 : 2 - 8 - 18 - 18 - 4 Group 4 Group 4A 14
Sb 51 : 2 - 8 - 18 - 18 - 5 Group 5 Group 5A 15
Te 52 : 2 - 8 - 18 - 18 - 6 Group 6 Group 6A 16
I 53 : 2 - 8 - 18 - 18 - 7 Group 7 Group 7A 17
Xe 54 : 2 - 8 - 18 - 18 - 8 Group 8 Group 8A 18
Cs 55 : 2 - 8 - 18 - 18 - 8 - 1 Group 1 Group 1A 1
Ba 56 : 2 - 8 - 18 - 18 - 8 - 2 Group 2 Group 2A 2
Lanterides : Rare Earth Metals : f-block
(Third electron layer from the outside is increasing. the 2 outer layers are stabile)
La 57 : 2 - 8 - 18 - 18 - 9 - 2 Group (19) Group - (3C)
Ce 58 : 2 - 8 - 18 - 19 - 9 - 2 Group (20) Group - (4C)
Pr 59 : 2 - 8 - 18 - 21 - 8 - 2 Group (21) Group - (5C)
Nd 60 : 2 - 8 - 18 - 22 - 8 - 2 Group (22) Group - (6C)
Pm 61 : 2 - 8 - 18 - 23 - 8 - 2 Group (23) Group - (7C)
Sm 62 : 2 - 8 - 18 - 24 - 8 - 2 Group (24) Group - (8C)
Eu 63 : 2 - 8 - 18 - 25 - 8 - 2 Group (25) Group - (9C)
Gd 64 : 2 - 8 - 18 - 25 - 9 - 2 Group (26) Group - (10C)
Tb 65 : 2 - 8 - 18 - 27 - 8 - 2 Group (27) Group - (11C)
Dy 66 : 2 - 8 - 18 - 28 - 8 - 2 Group (28) Group - (12C)
Ho 67 : 2 - 8 - 18 - 29 - 8 - 2 Group (29) Group - (13C)
Er 68 : 2 - 8 - 18 - 30 - 8 - 2 Group (30) Group - (14C) 1C ...!!!! (?)
Tm 69 : 2 - 8 - 18 - 31 - 8 - 2 Group (31) Group - (15C) 2C ...!!!! (?)
Yb 70 : 2 - 8 - 18 - 32 - 8 - 2 Group (32) Group - (16C) last f-block
Lu 71 : 2 - 8 - 18 - 32 - 9 - 2 Group (33) Group - (17C) (? no)
Transition Metals : d-block
Lu Group 9 Group 3B (correct group)
Hf 72 : 2 - 8 - 18 - 32 - 10 - 2 Group 10 Group 4B
Ta 73 : 2 - 8 - 18 - 32 - 11 - 2 Group 11 Group 5B
W 74 : 2 - 8 - 18 - 32 - 12 - 2 Group 12 Group 6B
Re 75 : 2 - 8 - 18 - 32 - 13 - 2 Group 13 Group 7B
Os 76 : 2 - 8 - 18 - 32 - 14 - 2 Group 14 Group 8B (a)
Ir 77 : 2 - 8 - 18 - 32 - 15 - 2 Group 15 Group 8B (b)
Pt 78 : 2 - 8 - 18 - 32 - 17 - 1 Group 16 Group 8B (c)
Au 79 : 2 - 8 - 18 - 32 - 18 - 1 Group 17 Group 1B
Hg 80 : 2 - 8 - 18 - 32 - 18 - 2 Group 18 Group 2B
Basic elements
Tl 81 : 2 - 8 - 18 - 32 - 18 - 3 Group 3 Group 3A
Pb 82 : 2 - 8 - 18 - 32 - 18 - 4 Group 4 Group 4A
Bi 83 : 2 - 8 - 18 - 32 - 18 - 5 Group 5 Group 5A
Po 84 : 2 - 8 - 18 - 32 - 18 - 6 Group 6 Group 6A
At 85 : 2 - 8 - 18 - 32 - 18 - 7 Group 7 Group 7A
Rn 86 : 2 - 8 - 18 - 32 - 18 - 8 Group 8 Group 8A
Fr 87 : 2 - 8 - 18 - 32 - 18 - 8 - 1 Group 1 Group 1A
Ra 88 : 2 - 8 - 18 - 32 - 18 - 8 - 2 Group 2 Group 2A
Actinides : Radioactive elements : f-block
Ac 89 : 2 - 8 - 18 - 32 - 18 - 9 - 2 Group (19) Group - (3C)
Th 90 : 2 - 8 - 18 - 32 - 18 - 10- 2 Group (20) Group - (4C)
Pa 91 : 2 - 8 - 18 - 32 - 18 - 11- 2 Group (21) Group - (5C)
U 92 : 2 - 8 - 18 - 32 - 21 - 9 - 2 Group (22) Group - (6C)
Np 93 : 2 - 8 - 18 - 32 - 22 - 9 - 2 Group (23) Group - (7C)
Pu 94 : 2 - 8 - 18 - 32 - 24 - 8 - 2 Group (24) Group - (8C)
Am 95 : 2 - 8 - 18 - 32 - 25 - 8 - 2 Group (25) Group - (9C)
Cm 96 : 2 - 8 - 18 - 32 - 25 - 9 - 2 Group (26) Group - (10C)
Bk 97 : 2 - 8 - 18 - 32 - 27 - 8 - 2 Group (27) Group - (11C)
Cf 98 : 2 - 8 - 18 - 32 - 28 - 8 - 2 Group (28) Group - (12C)
Es 99 : 2 - 8 - 18 - 32 - 29 - 8 - 2 Group (29) Group - (13C)
Fm 100 : 2 - 8 - 18 - 32 - 30 - 8 - 2 Group (30) Group - (14C)
Md 101 : 2 - 8 - 18 - 32 - 31 - 8 - 2 Group (31) Group - (15C)
No 102 : 2 - 8 - 18 - 32 - 32 - 8 - 2 Group (32) Group - (16C) Last f-block
Lr 103 : 2 - 8 - 18 - 32 - 32 - 8 - 3 Group (33) Group - (17C) (? no)
Transition Metals : d-block
Lr Group 9 Group 3B (correct group)
Rf 104 : 2 - 8 - 18 - 32 - 32 - 10 -2 Group 10 Group 4B
Db 105 : 2 - 8 - 18 - 32 - 32 - 11 -2 Group 11 Group 5B
Sg 106 : 2 - 8 - 18 - 32 - 32 - 12 -2 Group 12 Group 6B
Bh 107 : 2 - 8 - 18 - 32 - 32 - 13 -2 Group 13 Group 7B
Hs 108 : 2 - 8 - 18 - 32 - 32 - 14 -2 Group 14 Group 8B (a)
Mt 109 : 2 - 8 - 18 - 32 - 32 - 15 -2 Group 14 Group 8B (b)
Ds 110 : 2 - 8 - 18 - 32 - 32 - 16 -2 Group 15 Group 8B (c)
Rg 111 : 2 - 8 - 18 - 32 - 32 - 17 -2 Group 16 Group 1B
Cn 112 : 2 - 8 - 18 - 32 - 32 - 18 -2 Group 17 Group 2B
Basic elements : P-block
Uut113 : 2 - 8 - 18 - 32 - 32 - 18 -3 Group 3 Group 3A
Fl 114 : 2 - 8 - 18 - 32 - 32 - 18 -4 Group 4 Group 4A
Uup115 : 2 - 8 - 18 - 32 - 32 - 18 -5 Group 5 Group 5A
Lv 116 : 2 - 8 - 18 - 32 - 32 - 18 -6 Group 6 Group 6A
Uus117 : 2 - 8 - 18 - 32 - 32 - 18 -7 Group 7 Group 7A
Uuo118 : 2 - 8 - 18 - 32 - 32 - 18 -8 Group 8 Group 8A
Uue119 : 2 - 8 - 18 - 32 - 32 - 18 -8 -1 Group 1 Group 1A
Ubn120 : 2 - 8 - 18 - 32 - 32 - 18 -8 -2 Group 2 Group 2A
Hypothetical : G-Block : sharing the spokes of f-block (?)
Ubu121 : 2 - 8 - 18 - 32 - 32 - 18 - 8 -3 Group (33) Group - (3D)
Ubb122 : 2 - 8 - 18 - 32 - 32 - 18 - 8 -4 Group (34) Group - (4D)
The Elements of the periodic table is the basis fotr all chemistry, electricity and material that we encounter on a familiar and daily basis. However the nature of these matters are surely stimulating the soul on the deepest level. An example of extraordinary beauty and complicated designis to see for instance a Bismuth Crystal, look it up and be thrilled !
The idea on this spiral design came from the investigation wether soft Iron that is more mallable and workable, and also have increased Magnetic respons. Thus being favoured in electrical generator coils. Wanting to buy soft Iron is a challenge. the industry wants Hard Iron that can take alot of wheight, not Iron. Hard Iron was in the old days wrought in a furnace and in that process of being heated adding coal paricles. Becoming Steel. steel is Hard Iron. Becoming Hard Iron the Fe26 becomes "contaminated and less Magnetically permable as it is called.
I asked myself if Stainless Steel that is another version of Iron Fe26 and C6 Carbon had such an amount of Carbone that the Oxygene O8 in the water and atmosphere did not React with the Iron, becoming Rust or Oxidized ? It was not so the stainless Steel is an alloy that has more atomic elements than just Carbon. It consist of Chromium 24, Manganese 25, Silicon 14 and Nickel 14. these metals form a "seal" over the Iron atoms not allowing the Oxygene that is a strong Acid atom to react and Oxidize forming Rust on the Iron atom. Oxygene is a greek word meaning OXY (sour) and Genes gender . Oxygene is very electron rich in its placement in the octet of the periodic table.
The Octet Rule.
The Electrons are in their nature Negative, and the Nucleus UUD is in their nature of opposite polarity +. Making the atom a magentic Dipole. dipole means 2 - poles, North and South considered a "magnet pair". when the electrons are rubbed away from the Atom, it becomes Charged, that is known in Triboelectric Charge when for instance a PVC pipe is charged (+) poisitve by rubbing a cloth onto it. the electrons are removed, leaving the Atoms in need of a need for balance.
The Atom Octet has an "invisible desire" that in the desires a 2, 8, 18, or 32 electrons in the outer shells. when the innermost electron shells are stabile. The different octet domains are 2 electrons in the outer shell, or 8 in the next 18, then 32 electrons in the outermost shell. Lithium for example has 3 protons and 3 neutrons but still desires 8 Electrons in its outer shell. the Atoms can (seemingly) interact in reactions where all the 8 electrons are utilized in a combination with other atoms, forming a molecule. This is called the Covalent Bond theory and well established in the chemical community.
I am still investigating Molecules and how the charges of electrons apply to the Protons. It will take some time. so before all that is in place I just print the Spiral table to get som comments on it.
I was very proud of it at once, then soon realizing it is made a spiral Periodic system back in the days by Ingo Waldemar Dagobert Hackh 1914. so this is nothing new. However I have seen so many "periodic systems so this is but a new interpreteion. I will keep more coming when I find something interesting to write. The basis of this investigation is to find out what way the Electrons move in a DC battery. Do they go from + to -. Or from negative - to positive + ? This particular model is more concerned with the Atoms and the electrons. It is more complex when it comes to molecules. and it will take more time to investigate.
The Spiral Periodic System.
It seemed reasonable to figure a way to make the Atoms progress along a spiral as the proton mass increased. the Groups are arranged in spokes from the center.
The Rendering is in Low resolution. But the idea is visible.
This is a early version. Write an Email and I will be happy to send a full resolution bitmap file.
The idea presented itself to me when I wanted to draw a spiral version
of the Period System. I drew the basic elemets on paper, then I saw
the limitations of a 8 symmetry. when Iron shurely did not go into group 8 along with the inert gases. The 8 symmetry had to depart at Calcium and enter a new "domain" Scandium. I expanded the design in a special way and suddenly I saw a large system unfold. Including the Actanides and the Lanthanides in a spiral system that did not have the loops or additional rows of elements in the classical designs. This design seems to function in a good way, it looks very neat and hopefully of inspiration. Here is what I have.
Kenneth Henrik Olsen.
Oslo 10.7.2013
---
Periodic Table Spiral Model.
A Design purpose model that neatly includes all the blocks
in a spiral expanding from a origo point.
In a peculiar angular division for each block secion.
All the Blocks depart from the P-Block at group 2A.
The division is as follows :
In this design Group 1A is a on the Horizontal line
and Group 2A is 45 degrees anticlockwise.
P-Block : Basic Elements. Have a 8 symmetry and 8 spokes on 360 degrees. Groups are separated by 45 degrees.
D-Block : Transistion metals have 10 symmetry on 360 + 45 = 405 degrees
from spoke 2A. 405 / 11 = 36,81 degrees between the spokes.
and spiral turn exit D block into P-block group 3A.
F-Block : Lanthanides and actinides have a 15 elements
thus a 15 symmetry spread over (36,81*2 ) + 360 = 433,62 degrees
From spoke 2A. 433,62 / 16 = 27,21 degrees between the elements (spokes).
The Spiral exits the F-Block into group 4B transition metals.
Both Lanthernides and Actinides share the same division and proceedure in this design. That is they share the same groups. A misunderstanding led me
to write Landanoids instead of Lanthanoids. I thought the name related to "Land" since it
is Rare Earth Metals. Now knowing that Lanthnoids
are greek from "hidden elements".
It is neccesary to divide the 10 elemetns of Fblock with
405 / 11, because the 11th spoke in the D-Block geometrically and graphically matches the p-block spoke, on the next spiral turn or "octave".
the same with the F-blocks that are 15 elements
they are divided by number 16. 433.62 / 16. the 16th is the "0th" spoke
on the next block, or d-block
---
The elements progress along the spiral as they aquire more
Protons and Neutrons in the core. The smalles atomic size
is therefore near the origo or central point of the spiral.
Ideally the Hydrogene and Helium should have their own 2 fold symmetry.
In this design the H and He share the 8 spoke symmetry of the P-block.
I Have taken the liberty to change the Group names for the
Heavier elements d-Block. 4C - 17C or group 19 - 33.
There is also a description on the Bases and Acids.
And a attempt to clearify the misunderstanding
that the Ampere moves from the + terminal of the battery toward the -.
Load up the drawing and start the viewing from the central Origo.
The spiral will take you through the complete Periodic System !
Have a good one
Here is the work file where the numbers are arranged
Proton : Electrons New CAS IUPAC Group
H 1 : 1 Group 1 Group 1A 1
He 2 : 2 Group 2 Group 2A 2
Li 3 : 2 - 1 Group 1 Group 1A 1
Be 4 : 2 - 2 Group 2 Group 2A 2
B 5 : 2 - 3 Group 3 Group 3A 13
C 6 : 2 - 4 Group 4 Group 4A 14
N 7 : 2 - 5 Group 5 Group 5A 15
O 8 : 2 - 6 Group 6 Group 6A 16
F 9 : 2 - 7 Group 7 Group 7A 17
Ne 10 : 2 - 8 Group 8 Group 8A 18
Na 11 : 2 - 8 - 1 Group 1 Group 1A 1
Mg 12 : 2 - 8 - 2 Group 2 Group 2A 2
Al 13 : 2 - 8 - 3 Group 3 Group 3A 13
Si 14 : 2 - 8 - 4 Group 4 Group 4A 14
P 15 : 2 - 8 - 5 Group 5 Group 5A 15
S 16 : 2 - 8 - 6 Group 6 Group 6A 16
Cl 17 : 2 - 8 - 7 Group 7 Group 7A 17
Ar 18 : 2 - 8 - 8 Group 8 Group 8A 18
K 19 : 2 - 8 - 8 - 1 Group 1 Group 1A 1
Ca 20 : 2 - 8 - 8 - 2 Group 2 Group 2A 2
Transition metals : d-block
Sc 21 : 2 - 8 - 9 - 2 Group 9 Group 3B 3
Ti 22 : 2 - 8 - 10 - 2 Group 10 Group 4B 4
V 23 : 2 - 8 - 11 - 2 Group 11 Group 5B 5
Cr 24 : 2 - 8 - 13 - 1 Group 12 Group 6B 6
Mn 25 : 2 - 8 - 13 - 2 Group 13 Group 7B 7
Fe 26 : 2 - 8 - 14 - 2 Group 14 Group 8B (a) 8
Co 27 : 2 - 8 - 15 - 2 Group 15 Group 8B (b) 9
Ni 28 : 2 - 8 - 16 - 2 Group 16 Group 8B (c) version 1 10
Ni 28 : 2 - 8 - 17 - 1 Group 16 Group 8B (c) version 2 10
Cu 29 : 2 - 8 - 18 - 1 Group 17 Group 1B 11
Zn 30 : 2 - 8 - 18 - 2 Group 18 Group 2B 12
Basic elements
Ga 31 : 2 - 8 - 18 - 3 Group 3 Group 3A 13
Ge 32 : 2 - 8 - 18 - 4 Group 4 Group 4A 14
As 33 : 2 - 8 - 18 - 5 Group 5 Group 5A 15
Se 34 : 2 - 8 - 18 - 6 Group 6 Group 6A 16
Br 35 : 2 - 8 - 18 - 7 Group 7 Group 7A 17
Kr 36 : 2 - 8 - 18 - 8 Group 8 Group 8A 18
Rb 37 : 2 - 8 - 18 - 8 - 1 Group 1 Group 1A 1
Sr 38 : 2 - 8 - 18 - 8 - 2 Group 2 Group 2A 2
Transition Metals : d-block
(Next to outer electron shell is increasing, outer is stabile at 1 electron)
Y 39 : 2 - 8 - 18 - 9 - 2 Group 9 Group 3B 3
Zr 40 : 2 - 8 - 18 - 10- 2 Group 10 Group 4B 4
Nb 41 : 2 - 8 - 18 - 12- 1 Group 11 Group 5B 5
Mo 42 : 2 - 8 - 18 - 13 -1 Group 12 Group 6B 6
Tc 43 : 2 - 8 - 18 - 13 -2 Group 13 Group 7B 7
Ru 44 : 2 - 8 - 18 - 15 -1 Group 14 Group 8B (a) 8
Rh 45 : 2 - 8 - 18 - 16 -1 Group 15 Group 8B (b) 9
Pd 46 : 2 - 8 - 18 - 18 Group 16 Group 8B (c) 10
Ag 47 : 2 - 8 - 18 - 18 - 1 Group 17 Group 1B 11
Cd 48 : 2 - 8 - 18 - 18 - 2 Group 18 Group 2B 12
Basic elements : P-block
( outer electron shell is increasing)
In 49 : 2 - 8 - 18 - 18 - 3 Group 3 Group 3A 13
Sn 50 : 2 - 8 - 18 - 18 - 4 Group 4 Group 4A 14
Sb 51 : 2 - 8 - 18 - 18 - 5 Group 5 Group 5A 15
Te 52 : 2 - 8 - 18 - 18 - 6 Group 6 Group 6A 16
I 53 : 2 - 8 - 18 - 18 - 7 Group 7 Group 7A 17
Xe 54 : 2 - 8 - 18 - 18 - 8 Group 8 Group 8A 18
Cs 55 : 2 - 8 - 18 - 18 - 8 - 1 Group 1 Group 1A 1
Ba 56 : 2 - 8 - 18 - 18 - 8 - 2 Group 2 Group 2A 2
Lanterides : Rare Earth Metals : f-block
(Third electron layer from the outside is increasing. the 2 outer layers are stabile)
La 57 : 2 - 8 - 18 - 18 - 9 - 2 Group (19) Group - (3C)
Ce 58 : 2 - 8 - 18 - 19 - 9 - 2 Group (20) Group - (4C)
Pr 59 : 2 - 8 - 18 - 21 - 8 - 2 Group (21) Group - (5C)
Nd 60 : 2 - 8 - 18 - 22 - 8 - 2 Group (22) Group - (6C)
Pm 61 : 2 - 8 - 18 - 23 - 8 - 2 Group (23) Group - (7C)
Sm 62 : 2 - 8 - 18 - 24 - 8 - 2 Group (24) Group - (8C)
Eu 63 : 2 - 8 - 18 - 25 - 8 - 2 Group (25) Group - (9C)
Gd 64 : 2 - 8 - 18 - 25 - 9 - 2 Group (26) Group - (10C)
Tb 65 : 2 - 8 - 18 - 27 - 8 - 2 Group (27) Group - (11C)
Dy 66 : 2 - 8 - 18 - 28 - 8 - 2 Group (28) Group - (12C)
Ho 67 : 2 - 8 - 18 - 29 - 8 - 2 Group (29) Group - (13C)
Er 68 : 2 - 8 - 18 - 30 - 8 - 2 Group (30) Group - (14C) 1C ...!!!! (?)
Tm 69 : 2 - 8 - 18 - 31 - 8 - 2 Group (31) Group - (15C) 2C ...!!!! (?)
Yb 70 : 2 - 8 - 18 - 32 - 8 - 2 Group (32) Group - (16C) last f-block
Lu 71 : 2 - 8 - 18 - 32 - 9 - 2 Group (33) Group - (17C) (? no)
Transition Metals : d-block
Lu Group 9 Group 3B (correct group)
Hf 72 : 2 - 8 - 18 - 32 - 10 - 2 Group 10 Group 4B
Ta 73 : 2 - 8 - 18 - 32 - 11 - 2 Group 11 Group 5B
W 74 : 2 - 8 - 18 - 32 - 12 - 2 Group 12 Group 6B
Re 75 : 2 - 8 - 18 - 32 - 13 - 2 Group 13 Group 7B
Os 76 : 2 - 8 - 18 - 32 - 14 - 2 Group 14 Group 8B (a)
Ir 77 : 2 - 8 - 18 - 32 - 15 - 2 Group 15 Group 8B (b)
Pt 78 : 2 - 8 - 18 - 32 - 17 - 1 Group 16 Group 8B (c)
Au 79 : 2 - 8 - 18 - 32 - 18 - 1 Group 17 Group 1B
Hg 80 : 2 - 8 - 18 - 32 - 18 - 2 Group 18 Group 2B
Basic elements
Tl 81 : 2 - 8 - 18 - 32 - 18 - 3 Group 3 Group 3A
Pb 82 : 2 - 8 - 18 - 32 - 18 - 4 Group 4 Group 4A
Bi 83 : 2 - 8 - 18 - 32 - 18 - 5 Group 5 Group 5A
Po 84 : 2 - 8 - 18 - 32 - 18 - 6 Group 6 Group 6A
At 85 : 2 - 8 - 18 - 32 - 18 - 7 Group 7 Group 7A
Rn 86 : 2 - 8 - 18 - 32 - 18 - 8 Group 8 Group 8A
Fr 87 : 2 - 8 - 18 - 32 - 18 - 8 - 1 Group 1 Group 1A
Ra 88 : 2 - 8 - 18 - 32 - 18 - 8 - 2 Group 2 Group 2A
Actinides : Radioactive elements : f-block
Ac 89 : 2 - 8 - 18 - 32 - 18 - 9 - 2 Group (19) Group - (3C)
Th 90 : 2 - 8 - 18 - 32 - 18 - 10- 2 Group (20) Group - (4C)
Pa 91 : 2 - 8 - 18 - 32 - 18 - 11- 2 Group (21) Group - (5C)
U 92 : 2 - 8 - 18 - 32 - 21 - 9 - 2 Group (22) Group - (6C)
Np 93 : 2 - 8 - 18 - 32 - 22 - 9 - 2 Group (23) Group - (7C)
Pu 94 : 2 - 8 - 18 - 32 - 24 - 8 - 2 Group (24) Group - (8C)
Am 95 : 2 - 8 - 18 - 32 - 25 - 8 - 2 Group (25) Group - (9C)
Cm 96 : 2 - 8 - 18 - 32 - 25 - 9 - 2 Group (26) Group - (10C)
Bk 97 : 2 - 8 - 18 - 32 - 27 - 8 - 2 Group (27) Group - (11C)
Cf 98 : 2 - 8 - 18 - 32 - 28 - 8 - 2 Group (28) Group - (12C)
Es 99 : 2 - 8 - 18 - 32 - 29 - 8 - 2 Group (29) Group - (13C)
Fm 100 : 2 - 8 - 18 - 32 - 30 - 8 - 2 Group (30) Group - (14C)
Md 101 : 2 - 8 - 18 - 32 - 31 - 8 - 2 Group (31) Group - (15C)
No 102 : 2 - 8 - 18 - 32 - 32 - 8 - 2 Group (32) Group - (16C) Last f-block
Lr 103 : 2 - 8 - 18 - 32 - 32 - 8 - 3 Group (33) Group - (17C) (? no)
Transition Metals : d-block
Lr Group 9 Group 3B (correct group)
Rf 104 : 2 - 8 - 18 - 32 - 32 - 10 -2 Group 10 Group 4B
Db 105 : 2 - 8 - 18 - 32 - 32 - 11 -2 Group 11 Group 5B
Sg 106 : 2 - 8 - 18 - 32 - 32 - 12 -2 Group 12 Group 6B
Bh 107 : 2 - 8 - 18 - 32 - 32 - 13 -2 Group 13 Group 7B
Hs 108 : 2 - 8 - 18 - 32 - 32 - 14 -2 Group 14 Group 8B (a)
Mt 109 : 2 - 8 - 18 - 32 - 32 - 15 -2 Group 14 Group 8B (b)
Ds 110 : 2 - 8 - 18 - 32 - 32 - 16 -2 Group 15 Group 8B (c)
Rg 111 : 2 - 8 - 18 - 32 - 32 - 17 -2 Group 16 Group 1B
Cn 112 : 2 - 8 - 18 - 32 - 32 - 18 -2 Group 17 Group 2B
Basic elements : P-block
Uut113 : 2 - 8 - 18 - 32 - 32 - 18 -3 Group 3 Group 3A
Fl 114 : 2 - 8 - 18 - 32 - 32 - 18 -4 Group 4 Group 4A
Uup115 : 2 - 8 - 18 - 32 - 32 - 18 -5 Group 5 Group 5A
Lv 116 : 2 - 8 - 18 - 32 - 32 - 18 -6 Group 6 Group 6A
Uus117 : 2 - 8 - 18 - 32 - 32 - 18 -7 Group 7 Group 7A
Uuo118 : 2 - 8 - 18 - 32 - 32 - 18 -8 Group 8 Group 8A
Uue119 : 2 - 8 - 18 - 32 - 32 - 18 -8 -1 Group 1 Group 1A
Ubn120 : 2 - 8 - 18 - 32 - 32 - 18 -8 -2 Group 2 Group 2A
Hypothetical : G-Block : sharing the spokes of f-block (?)
Ubu121 : 2 - 8 - 18 - 32 - 32 - 18 - 8 -3 Group (33) Group - (3D)
Ubb122 : 2 - 8 - 18 - 32 - 32 - 18 - 8 -4 Group (34) Group - (4D)



Kommentarer